Firefighters and Breast Cancer

At a Glance
Women are not included in most studies of firefighters’ health. In our literature review on firefighters and cancer, only 3 out of 20 studies evaluated women firefighters’ cancer risk.
When they are included, the number of women is often too small to draw reliable conclusions. This is also true for racial and ethnic backgrounds among both male and female firefighters. Often, the numbers of Black, Latino, or Asian firefighters in studies are too low to evaluate if they face unique health risks.
Based on what we know from the few studies of women firefighters, cancer incidence appears elevated. Of the specific cancers studied, the incidence of cervical, thyroid, and bladder cancers was elevated at a statistically significant level. Breast cancer incidence also appeared elevated, although this was not statistically significant. No other specific cancers have been studied in women.
While studies show an elevated incidence of cancer in male firefighters, including brain, colon, and kidney cancers, as well as leukemia and Hodgkin’s and non-Hodgkin’s lymphoma, the same has not been studied in female firefighters.
What we are doing
Since 2013, BCPP has partnered with the Women Firefighters Biomonitoring Collaborative (WFBC), a project of the Women Workers Biomonitoring Collaborative (WWBC). The WFBC, comprised of firefighters from SFFCPF and UFSW, researchers from the University of California, Berkeley, the University of California, San Francisco (UCSF), and Silent Spring Institute, and advocates from BCPP and Commonweal, collected blood and urine from firefighters in 2014. This collaboration led to three studies of chemical exposure among fire fighters that show that fire fighters are impacted by chemical exposure at the cellular level.
Study 1: Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco
Study Takeaway: This study concluded that female firefighters are exposed to higher levels of some PFAS chemicals than office workers, suggesting that some exposures may be occupationally related.
Your Title Goes Here
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Read more about this study
What Was Done: Researchers analyzed the blood and urine samples for the presence of PFAS and other chemicals of concern in the article Exposure to Perfluoroalkyl Substances in a Cohort of Women Firefighters and Office Workers in San Francisco.[1]
The study specifically measured exposures to PFAS, a common chemical in firefighting foams, household and commercial products, and flame retardants. Among women firefighters (N = 86) and office workers (N = 84) in San Francisco, serum samples were measured to compare PFAS levels between firefighters and office workers. Seven of 12 PFAS break-down products were detected in at least 70% of the study population, and four chemical breakdown products were detected in 100% of participants.

Study 2: Organophosphate and Organohalogen Flame-Retardant Exposure and Thyroid Hormone Disruption in a Cross-Sectional Study of Female Firefighters and Office Workers from San Francisco
Thyroid hormones (T3 and T4) are important endocrine biomarkers of the early effect of potential relevance for breast cancer and other adverse health outcomes. Total T4 – thyroxine hormone made by the thyroid gland – is the sum of bound T4 (attached to blood plasma proteins) and free T4 (freely circulates and enters body tissues). Total T4 is important for maintaining homeostasis or balance in all body functions.
In this study, Organophosphate and Organohalogen Flame-Retardant Exposure and Thyroid Hormone Disruption in a Cross-Sectional Study of Female Firefighters and Office Workers from San Francisco,[2] researchers asked the question: Does exposure to flame-retardant compounds change thyroid hormone levels?
Exposure to certain flame retardants has been associated with endocrine hormone disruption relevant to breast cancer[5][6] and breast tumor development in animal and limited human studies.[5][7][8] Prior studies have found that women firefighters have a higher incidence of and mortality from breast cancer than the general (nonfirefighter) population.[9-11] Additionally, in epidemiology studies, thyroid dysfunction is associated with increased cancer risk, including breast cancer.[12-14]
Understanding women firefighters’ exposure to chemicals linked to cancer and endocrine biomarkers, such as thyroid hormone disruption, can inform timely prevention efforts.

In summary, the study provides evidence that 1) women firefighters have higher levels of flame-retardant metabolites compared to office workers, and 2) high flame-retardant exposure in the form of a common bis(1,3-dichloro-2-propyl) phosphate (BDCPP) metabolite was associated with a decrease in thyroid hormone levels (total T4). Even small disruptions to thyroid hormones (such as the 2.88% decrease reported here) can have important adverse downstream health effects,[19] even within normal ranges or subclinical levels.[23] Thyroid dysfunction affects up to 5% of the population and is more likely to affect women than men.[21] Identifying environmental chemical exposures that may be associated with biological changes, such as thyroid disruption, could be relevant to adverse health outcomes such as cardiovascular disease,[16] thyroid disease,[18] brain development of offspring during gestation, and long-term adverse health outcomes such as breast cancer and other forms of cancer.[17]
Your Title Goes Here
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Read more about this study
Occupational exposures are understudied in women. More research is needed on women firefighters because they are highly exposed to chemicals through fire and are understudied in the scientific literature. Examples include polyaromatic hydrocarbons, formaldehyde, benzene, dioxins, diesel, per- and poly-fluoroalkyl substances (PFASs), and flame retardants (FRs), including organohalogen FRs, such as polybrominated diphenyl ethers (PBDEs), and organophosphate FRs (OPFRs).
Research Question: Occupational exposure to flame retardants (FRs), a class of suspected endocrine-disrupting compounds, is a health concern for firefighters. Concerns about toxicity, bioaccumulation (accumulation in tissue over time), and long half-life of PBDEs (previously used flame retardants) led to an increase in the use of replacement flame retardants in the early 2000s, including organophosphate flame retardants (OPFRs), in furniture, fabrics, and electronics.[4] Furniture and electronics in homes and offices can be a reservoir for both legacy and replacement FRs; many of these products are not frequently replaced, and the chemicals applied to them are relatively stable, enabling these compounds to persist in homes and offices for decades.[22]
Exposure to OPFRs has been associated with endocrine disruption relevant to breast cancer[5],[6], as well as breast tumor development in animal studies and limited human studies.[5],[7],[8] Prior studies have found that women firefighters have a higher incidence of and mortality from breast cancer than the general (nonfirefighter) population.[9-11] Additionally, in epidemiology studies, thyroid dysfunction is associated with increased cancer risk, including breast cancer.[12-14] Understanding women firefighters’ exposure to cancer-linked chemicals and biomarkers of effect, such as thyroid hormone disruption, can inform timely prevention efforts.
To ensure that researchers were studying the biological impacts of exposure to flame retardants rather than differences due to sociodemographic and health factors, certain factors were controlled for in the analysis, including race/ethnicity, BMI, educational attainment, occupation, and creatinine. Researchers assessed the relationship between FR exposures and eating certain foods and packaged foods based on prior literature suggesting an association with FR exposures.[20] The study excluded the participants without thyroid hormone measurements or who reported taking thyroid hormone replacement medications.
Researchers measured eight flame retardants in the urine of study participants. Then, they measured thyroid levels in blood samples. They measured TSH and total T4 in plasma because environmental chemicals have been shown to interact with thyroid hormone transport proteins and facilitate metabolism and excretion of the thyroid hormone from the body.[19] Alterations to total T4 may also be an indicator of biological perturbations.[15]
Comparing firefighters (n=84) to office workers (n=81), researchers found that firefighters had higher average levels of flame-retardant metabolites than office workers for all eight, with the largest median differences observed for DBuP, BDCPP, and BCEP. Firefighters had five times higher bis(1,3-dichloro-2-propyl) phosphate than office workers. Two times as much bis(1,3-dichloro-2-propyl) phosphate resulted in a 2.88% decrease in thyroid hormone (total thyroxine).
Researchers found that having a BMI of 25-29, compared to 18.5-24.9, was associated with increased levels of BDCPP, BCEP, and DBuP. This may be due to the fat-soluble nature of these compounds. Likewise, completing a bachelor’s degree or higher was associated with elevated FRs compared to those who completed some college or less. Race and ethnicity were not associated with most OPFRs except for BDCPP, where Black participants had a GM 2.52x (95% CI: 1.10, 5.75) higher than White participants. However, this may be due to the limited number of Black participants in the firefighter and office worker groups. There was no association between food consumption and eating packaged foods with flame retardants in either firefighters or office workers.
Although we observed small changes in total T4 levels, small disruptions to thyroid hormones can have important adverse downstream health effects,[19] even within normal ranges or subclinical levels.[23] Thyroid dysfunction affects up to 5% of the population and is more likely to affect women than men. [21] Identifying environmental chemical exposures that may be associated with biological changes, such as thyroid disruption, could be relevant to adverse health outcomes such as cardiovascular disease,[16] thyroid disease,[18] brain development of offspring during gestation, and long-term adverse health outcomes such as breast cancer and other types of cancer.[17]

Study 3: Associations between poly-fluoroalkyl substance and organophosphate flame-retardant exposures and telomere length in a cohort of women firefighters and office workers in San Francisco
Researchers conducted a third study called Associations between poly-fluoroalkyl substance and organophosphate flame-retardant exposures and telomere length in a cohort of women firefighters and office workers in San Francisco,[3] in which they examined alterations in telomere length. Telomeres are like caps at the end of DNA sequencing strands on each chromosome that protect the chromosome from damage. Telomeres become shorter each time the cell divides, so telomere shortening is a natural outcome of age. They can also be shortened by stress, smoking, obesity, lack of exercise, and a poor diet.[24-27]Shorter telomeres may increase genetic instability, while persistent telomere lengthening may promote deleterious cell survival and proliferation,[39, 54-56] such as in breast cancer.[50– 53]
Research Purpose: In this study, the researchers asked if certain chemical exposures were associated with telomere length in San Francisco, CA, women firefighters compared to office workers. They measured serum concentrations of polyfluoroalkyl substances (PFAS), urinary metabolites of flame retardants, including organophosphate flame retardants (OPFRs), and telomere length in peripheral blood leukocytes in women firefighters (N = 84) and office workers (N = 79).
Despite similarities in age, dairy consumption, and egg consumption, firefighters had longer telomeres than office workers. Out of 12 PFAS chemicals measured, perfluorohexane sulfonate (PFHxS) was found at significantly higher concentrations among firefighters than office workers. Exposure to two PFAS chemicals, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic (PFOS), was associated with significantly longer telomeres among the entire cohort.
Takeaway: The main study takeaways: 1). There were stronger and more significant associations between exposure to PFAS chemicals among firefighters compared to office workers. 2). Higher PFAS exposure was significantly associated with longer telomere length among firefighters.
Your Title Goes Here
Your content goes here. Edit or remove this text inline or in the module Content settings. You can also style every aspect of this content in the module Design settings and even apply custom CSS to this text in the module Advanced settings.
Read more about this study
PFAS are widely used to give grease, stain, and water resistance qualities to food packaging, non-stick cookware, paints, fabrics, carpets, and furniture.[72] However, they are of particular concern for firefighters’ health. Firefighters are exposed to PFAS when PFAS-containing products such as furniture and carpet combust (i.e., light on fire) and through firefighting gear and foams containing these compounds.[57, 58, 60, 61] Indeed, research shows firefighters have elevated concentrations of certain PFAS relative to non-firefighters.[58, 59, 61]
The International Agency for Research on Cancer (IARC) has designated the profession of firefighting as “possibly carcinogenic” (Group 2B).[28] Previous studies indicate that first responders and firefighters have elevated risk of various cancers, including brain, kidney, thyroid, breast, gastrointestinal, bladder, testicular, prostate, melanoma, lymphomas, and multiple myeloma.[29 -37] However, most of these studies have been conducted almost exclusively on men.
Shortened telomeres have been associated with many diseases, including certain cancers.[40-46] However, telomere lengthening has also been associated with cancer,[47-49] and there is evidence of an association between telomere lengthening and breast cancer.[50-53] Telomere shortening may increase genetic instability while lengthening may promote deleterious cell survival and proliferation.[39, 54-56]
For this study, samples were collected and analyzed for PFAS (from serum) and telomere length (whole blood leukocytes). To ensure that researchers were studying the biological impacts of chemical exposure rather than differences due to sociodemographic and health factors, covariates assessed include demographic variables such as race/ethnicity and education; health variables such as body mass index (BMI), stress, and sleep metrics; and food frequency variables. The final analysis was adjusted for age, occupation, the number of times dairy products were eaten per week, and the number of times eggs were eaten per week.
Research Question: Were chemical exposures associated with telomere length in women firefighters and office workers from San Francisco, CA?
Researchers measured serum concentrations of polyfluoroalkyl substances (PFAS), urinary metabolites of flame retardants, including organophosphate flame retardants (OPFRs), and telomere length in peripheral blood leukocytes in women firefighters (N = 84) and office workers (N = 79).
Results found that firefighters had longer telomeres than office workers but were otherwise similar to office workers in age, dairy consumption, and egg consumption. Out of 12 PFAS, 7 PFAS congeners had detection frequencies greater than 70%, of which four had detection frequencies of 100% (PFHxS, PFOA, PFOS, PFNA). PFHxS was found at significantly higher concentrations among firefighters compared to office workers. Higher concentrations of PFNA were also observed among firefighters. However, the group difference was not statistically significant in this subset of WWBC data. Distributions of the remaining PFAS were similar across groups.
Exposure to PFOA and PFOS was associated with significantly longer TL among the entire cohort. A doubling in PFOA was associated with a 240 (95% CI 25, 455) bp increase in TL. PFOS was associated with a 172 (95% CI 5, 340) bp increase in TL. In firefighters, PFOA was significantly associated with TL. A doubling of PFOA was associated with a 329 (95% CI 13,645) bp increase in TL.
It was hypothesized that some persistent chemicals activate the aryl hydrocarbon receptor (AhR), which up-regulates telomerase and may therefore promote cancer.[38] Telomerase activation is necessary for cell immortality, which is, in turn, necessary for tumorigenesis.[70] There is limited evidence of AhR activation by PFAS,[71] so telomerase activation may play an important role in the PFAS-telomerase relationship.
PFAS: Firefighters are exposed to PFAS through firefighting gear, firefighting foam, dust, and smoke on the job. Firefighters had slightly elevated levels of PFAS in their bodies compared to office workers, which may be associated with adverse health outcomes, including cancer.
Organophosphate and Organohalogen Flame-Retardant Compounds Study: Firefighters have higher detection of OPFR metabolites than office workers. FR metabolites may contribute to endocrine dysfunction and are associated with reduced levels of thyroid hormone thyroxine (T4).
Telomere Length Study: Telomeres are protective caps at the end of chromosomes. Telomeres lengthen during cancer growth activity through uncontrolled cell proliferation. OPFRs and PFAS exposure in firefighters are associated with longer telomeres.
Study 1. PFAS (congeners: PFNA, PFOA, PFDA, and PFUnDA).
Women firefighters are exposed to higher levels of some PFAS chemicals compared to office workers.
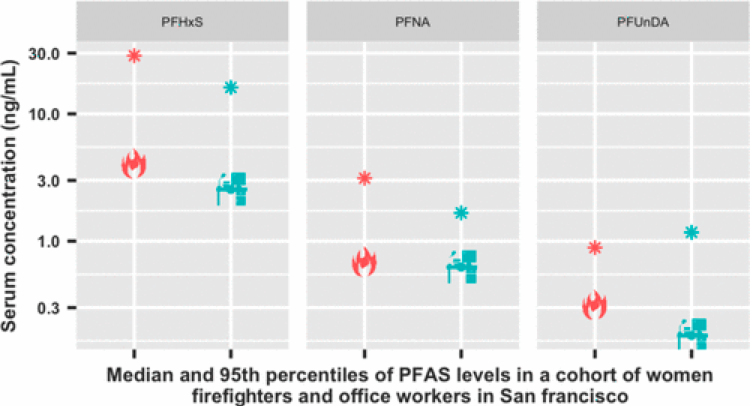
Source: https://pubs.acs.org/doi/abs/10.1021/acs.est.9b05490
Study 2. Organophosphate and Organohalogen Flame-Retardant compounds.
1) Women firefighters have higher levels of flame-retardant metabolites compared to office workers, and 2) high flame-retardant exposure in the form of a common bis(1,3-dichloro-2-propyl) phosphate (BDCPP) metabolite was associated with decrease in thyroid hormone levels (total T4).
Study 3. Results: 1). positive associations between PFAS and telomere length in women workers, with larger effects seen among firefighters compared to office workers. 2). The OPFR (organophosphate FRs) metabolites BDCPP and BCEP are also associated with longer telomere length in firefighters and office workers.
References
[1] Trowbridge, Jessica, Gerona, Roy R., Lin, Thomas, Rudel, Ruthann A., Bessonneau, Vincent, Buren, Heather, and Rachel Morello-Frosch. “Exposure to perfluoroalkyl substances in a cohort of women firefighters and office workers in San Francisco.” Environmental science & technology 54, no. 6 (2020): 3363. Accessed July 8, 2023. https://doi.org/10.1021/acs.est.9b05490.
[2] Trowbridge, Jessica, Gerona, Roy, McMaster, Michael, Ona, Katherine, Clarity, Cassidy, Bessonneau, Vincent, Rudel, Ruthann, Buren, Heather, and Rachel Morello-Frosch. “Organophosphate and Organohalogen Flame-Retardant Exposure and Thyroid Hormone Disruption in a Cross-Sectional Study of Female Firefighters and Office Workers from San Francisco.” Environmental Science & Technology 56, no. 1 (2022): 440-450. Accessed July 8, 2023. https://doi.org/10.1021/acs.est.1c05140.
[3] Clarity, Cassidy, Trowbridge, Jessica, Gerona, Roy, Ona, Katherine, McMaster, Michael, Bessonneau, Vincent, Rudel, Ruthann, Buren, Heather, and Rachel Morello-Frosch. “Associations between polyfluoroalkyl substance and organophosphate flame-retardant exposures and telomere length in a cohort of women firefighters and office workers in San Francisco.” MedRxiv,. Accessed July 8, 2023. https://doi.org/10.1101/2020.11.05.20226183.
[4] Van der Veen, Ike, and Jacob de Boer. “Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis.” Chemosphere 88, no. 10 (2012): 1119-1153. Accessed July 2, 2023. https://doi.org/10.1016/j.chemosphere.2012.03.067.
[5] Rudel, Ruthann A., Fenton, Suzanne E., Ackerman, Janet M., Euling, Susan Y., and Susan L. Makris. “Environmental Exposures and Mammary Gland Development: State of the Science, Public Health Implications, and Research Recommendations.” Environmental Health Perspectives 119, no. 8 (2011): 1053-1061. Accessed June 27, 2023. https://doi.org/10.1289/ehp.1002864.
[6] Gore, A. C., Chappell, V. A., Fenton, S. E., Flaws, J. A., Nadal, A., Prins, G. S., Toppari, J., and R. T. Zoeller. “EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals.” Endocrine Reviews 36, no. 6 (2015). Accessed June 27, 2023. https://doi.org/10.1210/er.2015-1010.
[7] Rodgers, Kathryn M., Udesky, Julia O., Rudel, Ruthann A., and Julia G. Brody. “Environmental chemicals and breast cancer: An updated review of epidemiological literature informed by biological mechanisms.” Environmental Research 160, (2018): 152-182.
Accessed June 27, 2023. https://doi.org/10.1016/j.envres.2017.08.045.
[8] Rudel, Ruthann A., Ackerman, Janet M., Attfield, Kathleen R., and Julia G. Brody. “New Exposure Biomarkers as Tools for Breast Cancer Epidemiology, Biomonitoring, and Prevention: A Systematic Approach Based on Animal Evidence.” Environmental Health Perspectives 122, no. 9 (2014): 881-895. Accessed June 27, 2023. https://doi.org/10.1289/ehp.1307455.
[9] Lee, David J., Koru-Sengul, Tulay, Hernandez, Monique N., Caban-Martinez, Alberto J., McClure, Laura A., Mackinnon, Jill A., and Erin N. Kobetz. “Cancer risk among career male and female Florida firefighters: Evidence from the Florida Firefighter Cancer Registry (1981-2014).” American Journal of Industrial Medicine 63, no. 4 (2020): 285-299. Accessed June 27, 2023. https://doi.org/10.1002/ajim.23086.
[10] Ma, Fangchao MD, PhD; Fleming, Lora E. MD, PhD; Lee, David J. PhD; Trapido, Edward ScD; Gerace, Terence A. PhD. Cancer Incidence in Florida Professional Firefighters, 1981 to 1999. Journal of Occupational and Environmental Medicine 48(9), (2006): 883-888. Accessed June 27, 2023. https://doi.org/10.1097/01.jom.0000235862.12518.04.
[11] Ma, Fangchao, Fleming, Lora E., Lee, David J., Trapido, Edward, Gerace, Terence A., Lai, Hong, and Shenghan Lai. “Mortality in Florida professional firefighters, 1972 to 1999.” American Journal of Industrial Medicine 47, no. 6 (2005): 509-517. Accessed June 27, 2023. https://doi.org/10.1002/ajim.20160.
[12] Angelousi, Anna, Evanthia Diamanti-Kandarakis, Evangelia Zapanti, Afroditi Nonni, Eftuxios Ktenas, Aimilia Mantzou, Konstantinos Kontzoglou, and Grigorios Kouraklis. “Is There an Association Between Thyroid Function Abnormalities and Breast Cancer?” Archives of Endocrinology and Metabolism 61, no. 1 (2017): 54–61. Accessed June 27, 2023. https://doi.org/10.1590/2359-3997000000191.
[13] Kim, Eun Y., Chang, Yoosoo, Lee, Kwan H., Yun, Sup, Park, Yong L., Park, Chan H., Ahn, Jiin, Shin, Hocheol, and Seungho Ryu. “Serum concentration of thyroid hormones in abnormal and euthyroid ranges and breast cancer risk: A cohort study.” International Journal of Cancer 145, no. 12 (2019): 3257-3266. Accessed June 27, 2023. https://doi.org/10.1002/ijc.32283.
[14] Khan, Samer R., Chaker, Layal, Ruiter, Rikje, Aerts, Joachim G., Hofman, Albert, Dehghan, Abbas, Franco, Oscar H., Stricker, Bruno H., and Robin P. Peeters. “Thyroid Function and Cancer Risk: The Rotterdam Study.” The Journal of Clinical Endocrinology & Metabolism 101, no. 12 (2016): 5030-5036. Accessed June 27, 2023. https://doi.org/10.1210/jc.2016-2104.
[15] Diamanti-Kandarakis, Evanthia, Bourguignon, Jean-Pierre, Giudice, Linda C., Hauser, Russ, Prins, Gail S., Soto, Ana M., Zoeller, Thomas R., and Gore, Andrea C. “Endocrine-Disrupting Chemicals: An Endocrine Society Scientific Statement.” Endocrine Reviews 30, no. 4 (2009): 293–342. Accessed July 6, 2023. https://doi.org/10.1210/er.2009-0002
[16] Rodondi, Aujesky, Vittinghoff, Cornuz, and Bauer D. “Subclinical Hypothyroidism and the Risk of Coronary Heart Disease: A Meta-Analysis.” AJM Theme Issue: Cardiology Review 119, no. 7 (2006): 541-551. Accessed June 27, 2023. https://doi.org/10.1016/j.amjmed.2005.09.028
[17] Krashin, Eilon, Piekiełko-Witkowska, Agnieszka, Ellis, Martin, and Osnat Ashur-Fabian. “Thyroid Hormones and Cancer: A Comprehensive Review of Preclinical and Clinical Studies.” Frontiers in Endocrinology 10, (2018). https://doi.org/10.3389/fendo.2019.00059.
[18] Ward, Jemal, and Chen A. “Increasing Incidence of Thyroid Cancer: Is Diagnostic Scrutiny the Sole Explanation?” Future Oncol. 6, no. (2010): 185–188. Accessed June 27, 2023. https://doi.org/10.2217/fon.09.161.
[19] Boas, Malene, Feldt-Rasmussen, Ulla, and Katharina M. Main. “Thyroid effects of endocrine disrupting chemicals.” Molecular and Cellular Endocrinology 355, no. 2 (2012): 240-248. Accessed June 27, 2023. https://doi.org/10.1016/j.mce.2011.09.005.
[20] Kim, Hyunju, Rebholz, Casey M., Wong, Eugenia, and Jessie P. Buckley. “Urinary organophosphate ester concentrations in relation to ultra-processed food consumption in the general US population.” Environmental Research 182, (2020): 109070. Accessed June 27, 2023. https://doi.org/10.1016/j.envres.2019.109070.
[21] Hollowell, Joseph G., Staehling, Norman W., Flanders, W. D., Hannon, W. H., Gunter, Elaine W., Spencer, Carole A., and Lewis E. Braverman. “Serum TSH, T4, and Thyroid Antibodies in the United States Population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III).” The Journal of Clinical Endocrinology & Metabolism 87, no. 2 (2002): 489-499. Accessed June 27, 2023. https://doi.org/10.1210/jcem.87.2.8182.
[22] Zota, Ami R., Linderholm, Linda, Park, June-Soo, Petreas, Myrto, Guo, Tan, Privalsky, Martin L., Zoeller, R. Thomas, and Woodruff, Tracey J. “Temporal Comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the Serum of Second Trimester Pregnant Women Recruited from San Francisco General Hospital, California.” Environmental Science & Technology 47, no. 20. (2013): 11776-11784. Accessed July 6, 2023. https://doi.org/10.1021/es402204y.
[23] Taylor, Peter N., Razvi, Salman, Pearce, Simon H., and Colin M. Dayan. “A Review of the Clinical Consequences of Variation in Thyroid Function Within the Reference Range.” The Journal of Clinical Endocrinology & Metabolism 98, no. 9 (2013): 3562-3571.
Accessed June 27, 2023. https://doi.org/10.1210/jc.2013-1315.
[24] Blackburn, Elizabeth H., and Elissa S. Epel. “Too toxic to ignore.” Nature 490, no. 7419 (2012): 169-171. Accessed June 30, 2023. https://doi.org/10.1038/490169a.
[25] Eisenberg, Dan T. A. “An evolutionary review of human telomere biology: The thrifty telomere hypothesis and notes on potential adaptive paternal effects.” American Journal of Human Biology 23, no. 2 (2011): 149-167. Accessed June 30, 2023. https://doi.org/10.1002/ajhb.21127.
[26] Aubert, Geraldine, and Lansdorp, Peter M. Accessed June 28, 2023. “Telomeres and Aging.” Physiological Reviews 88, (2008): 557-579. Accessed June 30, 2023. https://doi.org/00026-07.
[27] Ornish, Dean, Jue Lin, June M. Chan, Elissa Epel, Colleen Kemp, Gerdi Weidner, Ruth Marlin, et al. 2013. “Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study.” The Lancet Oncology 14, no. 11 (2013): 1112-1120. Accessed June 30, 2023. https://doi.org/10.1016/S1470-2045(13)70366-8.
[28] International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Painting, Firefighting, and Clarity et al. World Health Organization 98, (2010). Accessed July 7, 2023. https://monographs.iarc.fr/wp-content/uploads/2018/06/mono98.pdf.
[29] Daniels, D Robert D., Kubale, Travis L., Yiin, James H, Dahm, Matthew M., Hales, Thomas R., Baris, Dalsu, Zahm, Shelia H., Beaumont, James J., Waters, Kathleen M., and Pinkerton, Lynne E. “Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco,” Occup Environ Med. 71, no. 6 (2014):388–97. Accessed July 7, 2023. https://doi.org/10.1136/oemed-2013-101662.
[30] LeMasters, Grace K., Genaidy, Ash M., Succop, Paul, Deddens, James, Sobeih, Tarek, Barriera-Viruet, Heriberto, Dunning, Kari, Lockey, and James. “Cancer Risk Among Firefighters: A Review and Meta-analysis of 32 Studies.” Journal of Occupational and Environmental Medicine 48, no. 11 (2006):1189-1202. Accessed July 7, 2023. https://doi.org/10.1097/01.jom.0000246229.68697.90.
[31] Ahn, Yeon-Soon, Jeong, Kyoung-Sook, and Kim, Kyoo-Sang.” Cancer morbidity of professional emergency responders in Korea.” . Am J Ind Med. 55, no.9 (2012):768–778. Accessed July 7, 2023. https://doi.org/10.1002/ajim.22068.
[32] Bates, Michael N. “Registry-based case–control study of cancer in California firefighters.” Am J Ind Med. 50, no.5 (2007):339–344. Accessed July 7, 2023. https://doi.org/10.1002/ajim.20446.
[33] Delahunt B, Bethwaite PB, Nacey JN. “Occupational risk lor renal cell carcinoma. A case-control study based on the New Zealand Cancer registry.” Br J Urol. 75, no. 5 (1995):578–582. https://doi.org/10.1111/j.1464-410X.1995.tb07410.x.
[34] Dongmug Kang MD, PhD, Letitia K. Davis ScD, Phillip Hunt PhD, David Kriebel ScD. “Cancer incidence among male Massachusetts firefighters.” Am J Ind Med.51, no. 5 (2008):329– 335. Accessed July 7, 2023. https://doi.org/10.1002/ajim.20549.
[35] Ma, Fangchao, Fleming, Lora E., Lee, David J., Trapido, Edward, Gerace, Terence A., Lai, Hong, and Lai, Shenghan. “Mortality in Florida professional firefighters.” Am J Ind Med.47, no. 6 (2005): 509–517. Accessed July 7, 2023. https://doi.org/10.1002/ajim.20160.
[36] Tsai, Rebecca J., Luckhaupt, Sara E., Schumacher, Pam, Cress, Rosemary D., Deapen, Dennis M., and Calvert, Geoffrey M..“Risk of cancer among firefighters in California.” Am J Ind Med. 58, no.7 (2015):715–29. Accessed July 7, 2023. https://doi.org/10.1002/ajim.22466.
[37] Jalilian, Hamed, Ziaei, Mansour, Weiderpass, Elisabete, Silvia Rueegg, Corina, Khosravi, Yahya, and Kjaerheim, Kristina. “Cancer incidence and mortality among firefighters.” Int J Cancer. 145, no. 10 (2019):2639–2646. Accessed July 7, 2023. https://doi.org/10.1002/ijc.32199.
[38] Mitro, Susanna D., Birnbaum, Linda S., Needham, Belinda L., and Zota, Ami R. “Cross-sectional Associations between Exposure to Persistent Organic Pollutants and Leukocyte Telomere Length among U.S. Adults in NHANES.” Environ Health Perspect. 124, no. 5 (2016):651–658. Accessed July 7, 2023. https://doi.org/10.1289/ehp.1510187.
[39] Mathon, Nicole F., and Alison C. Lloyd. “Cell Senescence and Cancer.” Nature Reviews Cancer 1, no. 3 (2001): 203 – 213. Accessed July 7, 2023. https://doi.org/10.1038/35106045.
[40] Cawthon, Richard M., Smith, Ken R., O’Brien, Elizabeth, Sivatchenko, Anna, and Richard A Kerber. “Association between telomere length in blood and mortality in people aged 60 years or older.” Lancet 361, no. 9355 (2003):393–395. Accessed July 7, 2023. https://doi.org/10.1016/S0140-6736(03)12384-7.
[41] D’Mello, Matthew JJ., Ross, Stephanie A., Briel, Matthias, Anand, Sonia S., Gerstein, Hertzel, and Paré, Guillaume. “Association between shortened leukocyte telomere length and Cardiometabolic outcomes.” Circ Cardiovasc Genet. 8, no. 1 (2015):82– 90. Accessed July 7, 2023. https://doi.org/10.1161/CIRCGENETICS.113.000485.
[42] Haycock, Phillip C, Heydon, Emma E, Kaptoge, Stephen, Butterworth, Adam S, Thompson, Alex, and Willeit Peter. “Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis.” BMJ. 349, (2014): g4227. Accessed July 7, 2023. https://doi.Org/10.1136/bmj.g4227.
[43] Zhao, Jinzhao, Miao, Kun, Wang, Haoran, Ding Hu, and Wang Dao Wen. “Association between telomere length and type 2 diabetes mellitus: a Meta-analysis.” PLoS One. 8, no. 11 (2013): e79993. Accessed July 7, 2023. https://doi.org/10.1371/journal.pone.0079993.
[44] Cohen, Sheldon, Janicki-Deverts, Denise, Turner, Ronald B, Casselbrant, Margaretha L. et al. “Association between telomere length and experimentally induced upper respiratory viral infection in healthy adults.” JAMA. 309, no. 7 (2013): 699–705. Accessed July 7, 2023. https://doi.org/10.1001/jama.2013.613.
[45] Wentzensen, Ingrid M, Mirabello, Lisa, Pfeiffer, Ruth M, and Savage Sharon A. “The Association of Telomere Length and Cancer: A Meta-analysis.” Cancer Epidemiol Biomark Amp Prev. 20, no. 6 (2011):1238–50. Accessed July 7, 2023. https://doi.org/10.1158/1055-9965.EPI-11-0005.
[46] Ma, Hongxia, Zhou, Ziyaun, Wei, Sheng, Liu, Zhensheng, Pooley, Karen A., Dunning, Alison M, et al. “Shortened telomere length is associated with increased risk of Cancer: a Meta-analysis.” PLoS One. 6, no. 6 (2011): e20466. Accessed July 7, 2023. https://doi.org/10.1371/journal.pone.0020466.
[47] Zhang, Xiao, Lin, Shao, Funk, William E, and Hou, Lifang. “Environmental and occupational exposure to chemicals and telomere length in human studies.” Occup Environ Med. 70, no. 10 (2013):743–749. Accessed July 7, 2023. https://doi.org/10.1136/oemed-2012-101350.
[48] Caini, Saverio, Raimondi, Sara, Johansson, Harriet, De Giorgi, Vincenzo, Zanna, Ines, Palli, Domenico, and Gandini, Sara. “Telomere length and the risk of cutaneous melanoma and non-melanoma skin cancer: a review of the literature and meta-analysis.” J Dermatol Sci. 80, no. 3 (2015):168–174.Accessed July 7, 2023. https://doi.org/10.1016/j.jdermsci.2015.08.003.
[49] Haycock, Philip C., Burgess, Stephen, Nounu, Aayah, Zheng, Jie, et al. “Association between telomere length and risk of Cancer and non-neoplastic diseases: a Mendelian randomization study.” JAMA Oncol. 3, no. 5 (2017):636–651. Accessed July 7, 2023. https://doi.org/10.1001/jamaoncol.2016.5945.
[50] Pellatt, Andrew J., Wolff, Roger K., Torres-Mejia, Gabriela, John, Esther M., Herrick, Jennifer S., Lundgreen, Abbie, Baumgartner, Kathy B. et al. “Telomere Length, Telomere-Related Genes, and Breast Cancer Risk: The Breast Cancer Health Disparities Study.” Genes, chromosomes & cancer 52, no. 7 (2013). Accessed June 30, 2023. https://doi.org/10.1002/gcc.22056.
[51] Gramatges, Maria M., Melinda L. Telli, Raymond R. Balise, and James D. Ford. “Longer Relative Telomere Length in Blood from Women with Sporadic and Familial Breast Cancer Compared with Healthy Controls.” Cancer Epidemiology, Biomarkers & Prevention 19, no. 2 (2010): 605–613. Accessed June 30, 2023. https://doi.org/10.1158/1055-9965.epi-09-0896.
[52] Qu, Shimian, Wanqing Wen, Xiao-Ou Shu, Wong Ho Chow, Yong-Bing Xiang, Jie Wu, Bu-Tian Ji, et al. “Association of Leukocyte Telomere Length with Breast Cancer Risk: Nested Case-Control Findings From the Shanghai Women’s Health Study.” American Journal of Epidemiology 177, no. 7 (2013): 617–624. Accessed June 30, 2023. https://doi.org/10.1093/aje/kws291.
[53] Samavat, Hamed, Xiaoshuang Xun, Aizhen Jin, Renwei Wang, Woon-Puay Koh, and Jian-Min Yuan. “Association between Prediagnostic Leukocyte Telomere Length and Breast Cancer Risk: The Singapore Chinese Health Study.” Breast Cancer Research 21, no. 1 (2019). Accessed June 30, 2023. https://doi.org/10.1186/s13058-019-1133-0.
[54] Hackett, Jennifer, and Carol W. Greider. “Balancing Instability: Dual Roles for Telomerase and Telomere Dysfunction in Tumorigenesis.” Oncogene 21, no. 4 (January 21, 2002): 619–626. Accessed June 30, 2023. https://doi.org/10.1038/sj.onc.1205061.
[55] Hug, Nele, and Lingner, Joachim. “Telemere Length Homeostasis.” Chromosoma 115, no.6 (2006): 413-425. Accessed July 1, 2023. https://doi.org/10.1007/s00412-006-0067-3.
[56] Meena, Jitendra, Rudolph, Lenhard K., Günes, Cagatay. “Telomere Dysfunction, Chromosomal Instability and Cancer.” Chromosomal Instability in Cancer Cells 200, (2015): 61-79. Springer, Cham. https://doi.org/10.1007/978-3-319-20291-4_3.
[57] Laitinen, Juha A, Koponen, Jani, Koikkalainen, Janne, and Kiviranta, Hannu. “Firefighters’ exposure to perfluoroalkyl acids and 2-butoxyethanol present in firefighting foams.” Adv Biol Monit Occup Environ Health. 231, no. 2 (2014):227–32. https://doi.org/10.1016/j.toxlet.2014.09.007.
[58] Dobraca, Dina, Israel, Leslie, McNeel, Sandra, Voss, Robert, Wang, Miaomiao, Gajek, Ryszard, et al. “Biomonitoring in California firefighters: metals and perfluorinated chemicals.” J Occup Environ Med. 57, no. 1 (2015):88–97. Accessed July 7, 2023. https://doi.org/10.1097/JOM.0000000000000307.
[59] Trowbridge Jessica, Gerona Roy R, Lin Thomas, Rudel Ruthann A, Bessonneau Vincent, Buren Heather, et al. “Exposure to Perfluoroalkyl substances in a cohort of women firefighters and Office Workers in San Francisco.” Environ Sci Technol. 54, no. 6 (2020): 3363–3374. Accessed July 7, 2023. https://doi.org/10.1021/acs.est.9b05490.
[60] Moody, Cheryl A and Field, Jennifer A. “Perfluorinated surfactants and the environmental implications of their use in fire-fighting foams.” Environ Sci Technol. 34, no. 18 (2000):3864–3870. Accessed July 7, 2023. https://doi.org/10.1021/es991359u.
[61] Jin ChuanFeng, Sun YingHua, Islam Ahmed, Qian Yong, Ducatman Alan. “Perfluoroalkyl Acids Including Perfluorooctane Sulfonate and Perfluorohexane Sulfonate in Firefighters.” J Occup Environ Med. 53, no. 3 (2011):324-328. Accessed July 7, 2023. https://doi.org/10.1097/JOM.0b013e31820d1314.
[62] Barry, Vaughn, Winquist, Andrea, and Steenland, Kyle. “Perfluorooctanoic acid (PFOA) exposures and incident cancers among adults living near a chemical plant.” Environmental Health Perspectives 121, no. 11-12 (2013):1313–1318. Accessed July 7, 2023. https:2/doi.org/10.1289/ehp.1306615.
[63] Lopez-Espinosa, Jose, Mondal, Debapriya, Armstrong, Ben, Bloom, Michael S., and Tony Fletcher. “Thyroid Function and Perfluoroalkyl Acids in Children Living Near a Chemical Plant.” Environmental Health Perspectives 120, no. 7 (2012): 1036-1041. Accessed July 7, 2023. https://doi.org/10.1289/ehp.1104370.
[64] Lopez-Espinosa, Jose, Mondal, Debapriya, Armstrong, Ben G., Eskenazi, Brenda, and Tony Fletcher. “Perfluoroalkyl Substances, Sex Hormones, and Insulin-like Growth Factor-1 at 6–9 Years of Age: A Cross-Sectional Analysis within the C8 Health Project.” Environmental Health Perspectives 124, no. 8 (2016): 1269-1275. Accessed July 7, 2023. https://doi.org/10.1289/ehp.1509869.
[65] Liu, Han, Chen, Qian, Lei, Lei, Zhou, Wei, Huang, Lisu, Zhang, Jun, and Dan Chen. “Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances affects leukocyte telomere length in female newborns.” Environmental Pollution 235, (2018): 446-452. Accessed July 7, 2023. https://doi.org/10.1016/j.envpol.2017.12.095.
[66] Steenland, Kyle, Zhao, Liping, Winquist, Andrea, and Christine Parks. “Ulcerative Colitis and Perfluorooctanoic Acid (PFOA) in a Highly Exposed Population of Community Residents and Workers in the Mid-Ohio Valley.” Environmental Health Perspectives 121, no. 8 (2013): 900-905. Accessed July 7, 2023. https://doi.org/10.1289/ehp.1206449.
[67] Joensen, Ulla N., Bossi, Rossana, Leffers, Henrik, Jensen, Allan A., Skakkebæk, Niels E., and Niels Jørgensen. “Do Perfluoroalkyl Compounds Impair Human Semen Quality?” Environmental Health Perspectives 117, no. 6 (2009): 923-927. Accessed July 7, 2023. https://doi.org/10.1289/ehp.0800517.
[68] Bassler, John, Ducatman, Alan, Elliott, Meenal, Wen, Sijin, Wahlang, Banrida, Barnett, John, and Matthew C. Cave. “Environmental perfluoroalkyl acid exposures are associated with liver disease characterized by apoptosis and altered serum adipocytokines.” Environmental Pollution 247, (2019): 1055-1063. Accessed July 7, 2023. https://doi.org/10.1016/j.envpol.2019.01.064.
[69] Blake, Bevin E., Pinney, Susan M., Hines, Erin P., Fenton, Suzanne E., and Kelly K. Ferguson. “Associations between longitudinal serum perfluoroalkyl substance (PFAS) levels and measures of thyroid hormone, kidney function, and body mass index in the Fernald Community Cohort.” Environmental Pollution 242, (2018): 894-904. Accessed July 7, 2023. https://doi.org/10.1016/j.envpol.2018.07.042.
[70] Cong, Sheng, Wright, Woodring E., and Jerry W. Shay. “Human Telomerase and Its Regulation.” Microbiology and Molecular Biology Reviews 66, no. 3 (2002): 407-425. Accessed July 7, 2023. https://doi.org/10.1128/MMBR.66.3.407-425.2002.
[71] Long, Manhai, Ghisari, Mandana, and Bonefeld-Jørgensen Eva C. “Effects of perfluoroalkyl acids on the function of the thyroid hormone and the aryl hydrocarbon receptor.” Environ Sci Pollut Res. 20, no. 11 (2013):8045–8056. Accessed July 7, 2023. https://doi.org/10.1007/s11356-013-1628-7.
[72] Agency for Toxic Substances and Disease Registry. Toxicological Profile for Perfluoroalkyls [Internet]. 2018 Jun. Available from: https://www.atsdr.cdc.Gov/toxprofiles/tp200.pdf.
Types: Article, Fact Sheet




